| ABOUT US |  |
| FIND US |  |
| STATUTE |  |
| CHARTER |  |
| FINALITY |  |
DI BELLA FOUNDATION > Use of Octreotide
N.B. Keep the product refrigerated.
Octreotide is a somatostatine analogue comprising 8 amino acids, present also in the blood, that exerts inhibitory effects on the secretion of growth hormone (called somatotrope or GH). GH is principally responsible not only for human growth from childhood to adulthood, but also for tumor growth. It is also key in activating other strong and ubiquitous tumor growth factors like EGF (epidermal growth factor), VEGF (vascular endothelial growth factor), IGF1 (insulin-like growth factor), etc. As a result, the inhibition of GH - a mitogenic, potentially cancer-inductive factor - follows a clearly logical criterion, extensively documented in medical literature and based on clinical and trial evidence. Save for very few exceptions, failure to employ such anti-tumor molecule represents a break between scientific evidence and clinical practice in oncology, which rules it out. Octreotide can be employed either to strengthen the action of somatostatine-14 - administered subcutaneously during the night, 3 hours after dinner, by means of a rate – controlled infusion device - or as an alternative to somatostatine.
Octreotide is available in a 10 mg package, covering a period of 7-10 days, a 20 mg package covering 16-20 days, and a 30 mg package covering 25-28 days. Because the drug is long-acting, it can solidify easily if not properly diluted. Octreotide is intended to gradually dilute in the blood for the active ingredient to be slowly released. In fact, failure to carefully follow the instructions while preparing the solution may end up with the syringe getting clogged during injection, or the solution becoming solid during preparation. So, be sure to read all of the instructions before mixing the product:
Octreotide is a somatostatine analogue comprising 8 amino acids, present also in the blood, that exerts inhibitory effects on the secretion of growth hormone (called somatotrope or GH). GH is principally responsible not only for human growth from childhood to adulthood, but also for tumor growth. It is also key in activating other strong and ubiquitous tumor growth factors like EGF (epidermal growth factor), VEGF (vascular endothelial growth factor), IGF1 (insulin-like growth factor), etc. As a result, the inhibition of GH - a mitogenic, potentially cancer-inductive factor - follows a clearly logical criterion, extensively documented in medical literature and based on clinical and trial evidence. Save for very few exceptions, failure to employ such anti-tumor molecule represents a break between scientific evidence and clinical practice in oncology, which rules it out. Octreotide can be employed either to strengthen the action of somatostatine-14 - administered subcutaneously during the night, 3 hours after dinner, by means of a rate – controlled infusion device - or as an alternative to somatostatine.
Octreotide is available in a 10 mg package, covering a period of 7-10 days, a 20 mg package covering 16-20 days, and a 30 mg package covering 25-28 days. Because the drug is long-acting, it can solidify easily if not properly diluted. Octreotide is intended to gradually dilute in the blood for the active ingredient to be slowly released. In fact, failure to carefully follow the instructions while preparing the solution may end up with the syringe getting clogged during injection, or the solution becoming solid during preparation. So, be sure to read all of the instructions before mixing the product:
|
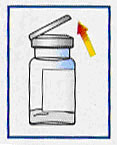
Take the product out of the fridge 15-20 minutes before time of use, to allow the vial and vehicle syringe to reach room temperature.
Remove cap from vial and assure that powder is settled at bottom of vial by lightly tapping vial. Failure to tap vial before injecting vehicle increases risk of clogging |

|
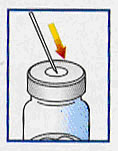
Remove cap from vehicle syringe and attach one of the supplied needles. |
|
Disinfect vial’s rubber stopper with alcohol swab and insert needle through centre. |
|
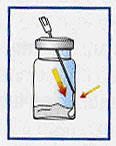
Without disturbing powder, gently inject vehicle into vial by running vehicle down inside wall of vial. Do not inject vehicle directly into powder. |
|
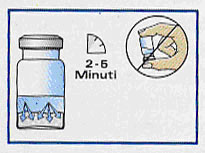
Do not disturb vial until vehicle has totally wetted powder (at least 2—5 minutes).
Without inverting vial, check sides and bottom of vial for dry spots.
If dry spots exist, allow undisturbed wetting to continue. Powder must be completely wetted before proceeding. |
|
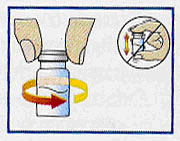
Once complete wetting has occurred,
vial should be moderately swirled for 30 to 60 seconds until a milky, uniform suspension is achieved. Do not invert or shake vial vigorously as this can cause lumps to form. |
|
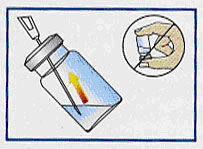
Immediately re-insert needle through rubber stopper and then—with bevel down and vial tipped at a 45-degree angle
(as shown in diagram)—slowly draw contents of vial into syringe.
Some residual suspension will remain—vial contains a calculated overfill. Never invert vial as this can affect the dose. |
|
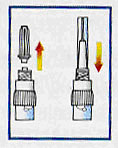
Discard first needle and attach second supplied needle. |

|
The drug must be administered immediately after suspension preparation. Continually rock syringe gently to maintain uniform suspension. Eliminate air from syringe. |
|
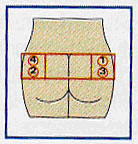
Disinfect injection site with alcohol swab. Insert needle deep into right or left gluteus, and if no blood vessel
has been penetrated, slowly inject suspension intra-muscularly (IM) with steady pressure. Octretide LAR must be given only by deep IM injection, never IV (intra-venously). If blood vessel is penetrated or if needle clogs, replace with new 1.1-mm diameter, 19-gauge needle and change injection site. |


